Radiation Treatment for Skin Cancer using the Axxent® Electronic Brachytherapy System® (eBx™)
Position Statement:
The Axxent® Electronic Brachytherapy System (eBx) and Axxent® Skin Applicator represent one option for treating Skin Cancer.
In the U.S. over 26,000 cases of skin cancer were treated with radiation therapy in 20061, primarily non-melanoma skin cancer (NMSC). Over a million NMSC cancers arise annually in the U.S., with basal cell carcinoma representing approximately 80% of cases and squamous cell carcinoma representing approximately 20%.
X-ray therapy has been an important treatment option for skin cancer for almost 100 years. Kilovoltage X-ray machines were commonly used for such treatments until the 1970s and 1980s, when kilovoltage machines gradually gave way to megavoltage linear accelerators. Currently radiation for skin cancer is typically delivered with electron beam therapy using megavoltage linear accelerators, or with HDR brachytherapy. Electron beam therapy and skin brachytherapy can be used interchangeably in many situations, but there are clearly defined advantages of HDR brachytherapy.
Although the prescription dose delivered using eBx is equivalent to that delivered with 192Ir, there are two characteristics which differentiate eBx from HDR delivered using 192Ir. First, there is no radioactive isotope; hence the detailed safety protocols and infrastructure to ensure radioactive source security are not required. Second, the lower energy of the Axxent® Minaturized X-ray Source allows the treatment to take place in a minimally shielded setting, decreasing radiation to providers and patients and increasing patient accessibility to brachytherapy.
Major Focus Points:
-
1. Radiation is an effective treatment modality for non-melanoma skin cancer.2
Radiation therapy has been employed successfully in the treatment of non-melanoma skin cancer since the early days of radiation therapy. It becomes the treatment of choice for cases in which tissues should be preserved for functional and/or cosmetic reasons and where radiotherapy appears to be a simpler solution than reconstructive surgery. Radiation is also indicated in patients with contraindications for such surgery, such as in the eyelids, median or lateral canthus of the eyes, nose, pinna of the ears, and lips3 . In addition, radiation therapy may represent the first choice of treatment for patients, who are medically unfit or refuse surgery, or where tumor infiltration and proximity to neural involvement limit required surgery.
The cure rates of non-melanoma skin cancer at 5 years after radiation therapy cited in the literature are generally very high and can be as high as 99%4,5,6,7 for small lesions with ≤ 2cm in greatest dimension (T1N0M0), and approximately 80% at 5 years for larger lesions of 2-5 cm in greatest dimension8,9,10,11,12.
The pinna of the ear accounts for 9-19%13 of all epithelial cancers and is an example for an anatomic site where radiation may be a simpler option than reconstructive surgery. In a retrospective study of 313 patients who were treated primarily with orthovoltage X-rays besides electrons, Silva14 showed that radiation therapy can effectively treat epithelial skin cancer of the pinna with actuarial control rates of 86.6% at 2 years and 79.2% at 5 years. This is comparable to results reported in the literature for radiation therapy of ear lesions, with local control rates ranging from 81-100%15,16,17,18 . Another end point, the actuarial 2- and 5-year grade 4 late toxicity rates, were reported as 4.9% and 7.3%, respectively, and are comparable to those found in other studies of radiation therapy for epithelial skin cancer of the pinna, in which necrosis rates were found to be between 0% and 13%19,15,16,17,18.
NMSC of the eyelids is an example in which kilovoltage X-ray therapy is preferable to electrons because of easy shielding, ability to minimize the field and elimination of the requirement for bolus20. The mean cure rate at 5 years from completion of radiotherapy of the eyelids is over 90%. Excellent functional and aesthetic results are routine, as well as extremely low incidence of side effects and long-term sequelae4,10,11,21,22. The area of the lacrimal duct and the internal canthus may represent a particular indication for radiation therapy because of the high risk of damage due to surgery3.
Radiation in the lower kilo-voltage range of 50 kV has been shown to deliver an effective dose to treat NMSC, with good cosmetic results23,24.
-
2. HDR has advantages in comparison to electron beam radiation therapy.
More precise set-up and smaller target field and aperture sizes are possible with a brachytherapy source due to sharper field edges (the 80-20 penumbral transition width is less than 3 mm for brachytherapy vs. 10-12 mm when using MeV electrons). In addition, the field can more easily be shaped when administering brachytherapy, using lead foil or lead equivalent shields, than with thick shielding blocks used with electron radiation. Also, it is more difficult to maneuver an 8000-pound linear accelerator unit to accurately target the lesion.
Externally accessible body cavities (e.g. oral, genitor-urinary) can also be treated more easily with HDR brachytherapy than with electron beam therapy.
-
3. System offers comparable target coverage for skin treatments compared to Iridium 192 with at least equivalent performance.
-
a. Substantial Equivalence Evaluation of eBx and Varian VariSource Remote Afterloader– FDA
The code of Federal Regulations and the final FDA guidance on the 510(k) paradigm states that Class II devices, such as X-ray radiation therapy systems, require pre-market notification [510(k)] to demonstrate that the device to be marketed is as safe and effective as a legally marketed device. This so-called “substantial equivalence” requires that a new X-ray radiation therapy system has (i) the same intended use as the currently marketed X-ray radiation therapy system (predicate device), (ii) has the same technological characteristics as the predicate device; or (iii) has different technological characteristics that do not raise new questions of safety and effectiveness. The FDA requires submission of bench test measurements of system performance including source characterization, dose delivery, applicator durability, etc.
The first commercially manufactured Axxent® Electronic Brachytherapy System with Axxent® Balloon Applicator received FDA 510(k) clearance for commercial distribution on December 22, 2005. The device demonstrated substantial equivalence to the previously approved Photoelectron Corporation Photon Radiosurgery System and Spherical Applicators, the Varian VariSource Remote Afterloader, and the Proxima Therapeutics Mammosite Radiation Therapy System. The review and approval by the FDA clearly demonstrates that the Axxent® technology and performance is substantially equivalent to the previously approved predicate devices.
Subsequently, in February 2009, the Axxent® Electronic Brachytherapy System with the use of the Axxent® Surface Applicator received FDA clearance for use of the product for surface brachytherapy.
-
b. System has dose profiles comparable to 192Ir HDR brachytherapy in the treatment range and a uniform beam profile at treatment depth.
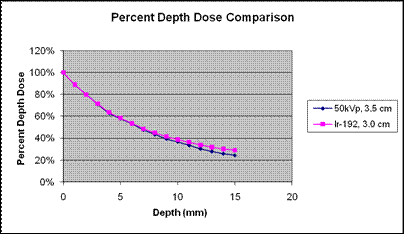
The Axxent® surface applicators have been designed to provide dose profiles that mimic Ir-192 profiles in the treatment range25 (Figure 1). The dose at the treatment depth is also uniform. The uniform dose at treatment depth is comparable to Ir-192 profiles when used with Valencia skin applicators26 (Figure 2), which are superior to typical Ir-192 dose profiles when used with Leipzig skin applicators27 . Both the system eBx applicators and the Valencia applictors for Ir-192 HDR employ flattening filters to achieve this dose uniformity.
Figure 1: Percent Depth Dose Comparison between 50 kV eBx and Ir-192 demonstrates comparable depth dose performance25,26,27. Also, the 50 kV dose falls off more steeply than Ir-192 beyond the target, reducing dose to non-target structures and reducing room shielding requirements.
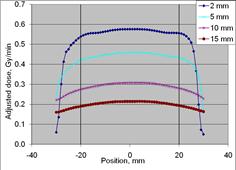
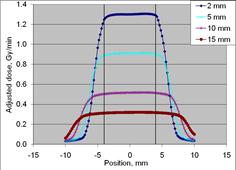
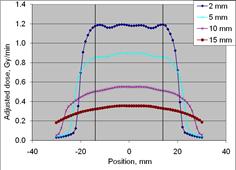
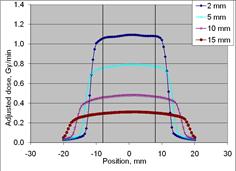
Figure 2: Dose profile measurements for eBx using the 10 mm Surface Applicator cone (upper left), 20 mm cone (upper right), 35 mm cone (lower left) and 50 mm cone (lower right) at four depths (2, 5, 10 and 15 mm). All cone sizes demonstrate uniformity at depth over approximately 80% of the field width. (dosimetry measurements25)
-
4. Electronic Brachytherapy has Advantages compared to HDR Brachytherapy with Iridium 192.
The Axxent® eBx system, utilizing a miniaturized X-ray tube, enables clinicians to administer HDR brachytherapy for skin lesions without the use of a radioactive isotope or a megavoltage linear accelerator. It can be performed at locations without requiring a heavily shielded room, due to minimal shielding required at these low energies with a faster dose fall-off28. As the therapists can remain at the patient side during treatment, visual monitoring of applicator set-up, procedure and patient during the treatment is possible and increases patient comfort and cooperation and improves targeting of the tumor. Due to the low kilo-voltage energy level, near ocular treatments are possible with thin tungsten eye-shields. On and off switch and quick stop capability of the controller provide increased procedural safety.
-
5. Greater accessibility of the eBx system will improve health care for patients and may ultimately reduce costs.
The eBx system uses low-energy kilovoltage radiation therapy and thus does not require a heavily shielded treatment room. In addition, the mobility of the system allows easy transport in between treatment rooms. The possibility to use eBx outside of large medical facilities, in satellite centers and physicians’ offices, brings brachytherapy treatments closer to the patient.
In addition, patient comfort can be increased with the use of eBx by allowing the therapist to stay in the room with the patient.
Conclusion:
Skin brachytherapy is an established treatment option with documented effectiveness and safety to treat non-melanoma skin cancer. Dosimetric measurements with the high-dose, low-energy Axxent® eBx system show comparable target doses for skin cancer treatments compared to 192Ir, and uniform dose at treatment depth. While providing the same target coverage as 192Ir, eBx allows better and continuous monitoring and more patient comfort due to the proximity of the therapist.
Greater accessibility in minimally shielded rooms and greater mobility of the system may enable more patients with skin cancer to receive radiation treatment, especially those who would not be candidates for another treatment option and would not be adequately treated.